A strategic approach towards DHF remediation of medical devices
Blog: Capgemini CTO Blog
Medical device regulations keep changing and they are becoming increasingly difficult to implement as the medical device industry evolves and adopts new technology faster than ever. One of the challenges medical devices manufacturers struggle with is planning for regulatory changes and acting on them in advance to avoid last-minute hassles. Regulations including FDA, EU MDR, EU IVDR are constantly changing and evolving. Regardless of the medical device’s lifecycle stage, the compliance issues can be a major setback. For legacy medical devices, it is a challenge to ensure that Design History Files (DHFs) for existing products are compliant with the new regulations.
We have seen that DHFs can become surprisingly complex documents as they are part of long-running development programs. A DHF is a compilation of records that describes the design history of a finished device. When remediation is required, it can pose distinct challenges to medical device manufacturers as they may not be fully prepared for this. Remediation of a quality system is a complex, time-consuming task that demands expertise and precise knowledge of procedures and risk management. Mergers and acquisitions and even change in manufacturing locations have also triggered the need to update design information files and other compliance documents for our clients.
DHF remediation includes a review of the design history files of legacy products to ensure compliance with quality system and regulatory requirements. New standards and regulations make it important to define an appropriate strategy to upgrade the DHF at the outset. With our experience, we have identified a simplistic approach to accommodate remediation for regulatory compliance.
Approach to remediation
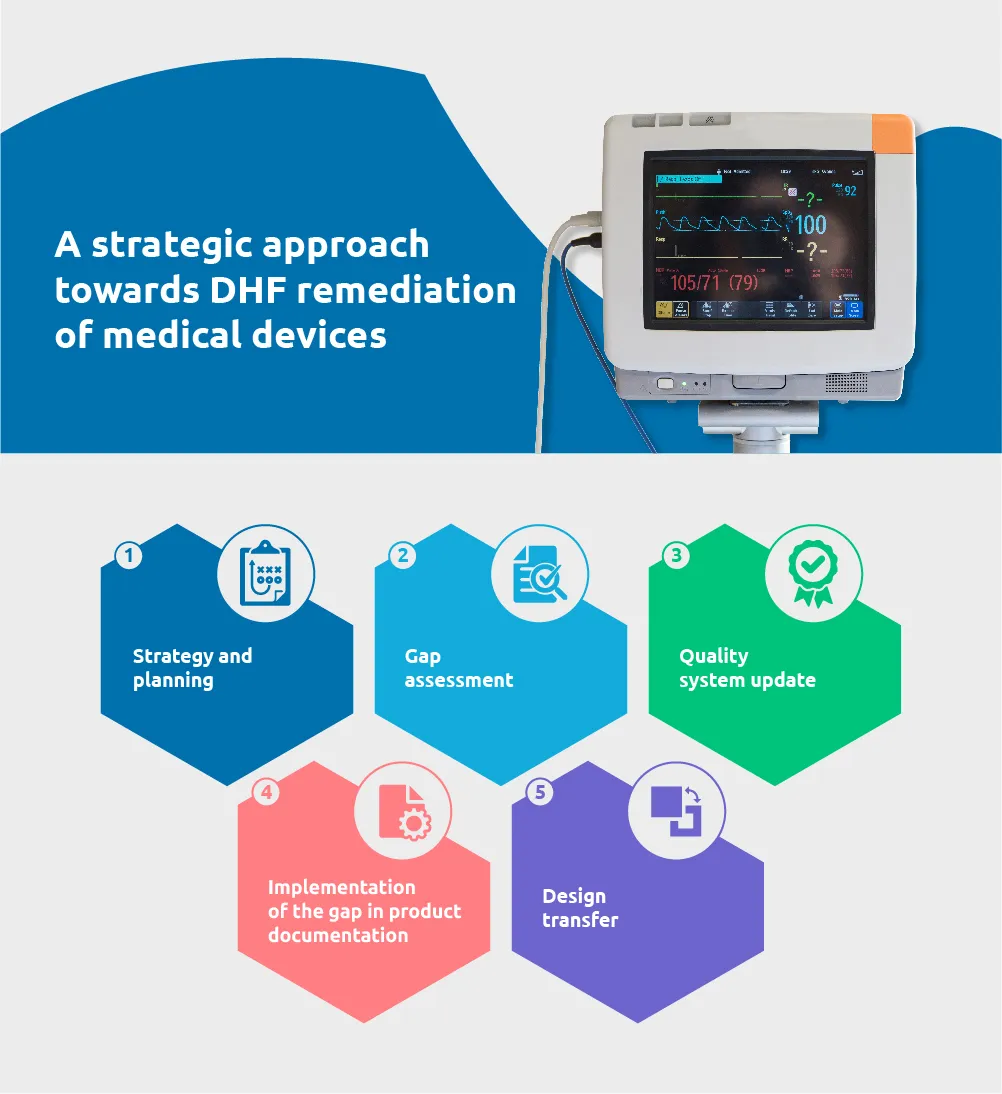
Strategy and planning
- Study of product portfolio and assess against the new regulations and future requirements
- Depending upon the need for remediation, plan a business strategy, along with the resources, training, budget, etc.
Gap assessment
- Quality system – gap assessment of SOP, WI, templates, tools for regulation and process standards
- DHF of every product – A systematic review of design history vis-à-vis a change triggered from regulation, quality system, etc. helps identify gaps in existing documentation
- Prepare a gap assessment report and a remediation plan.
Quality system update
- Revise standard operating procedures, work instructions, templates as identified in gap assessment.
Closing the gaps in product documentation
- Execute design controls process to generate or update the required product documentation
- Compile product documentation as per regulatory product documentation requirements
- Risk management file
- Usability engineering file
- Biological assessment document
- Design review
- Manufacturing information
- Applied standards list
- Safety and performance.
Design transfer
- Ensure correct design transfer into production specifications
A successful remediation project depends on many elements – a robust strategy, expert resources, and effective communication are vital aspects.
Reach out to learn more about how our industrialized approach to product remediation supports clients at an optimized cost and in a scalable, consistent, and transparent manner.
Visit our page to know more about remediation services for medical devices.