What is Change Management for Medical Devices?
Blog: Interneer blog
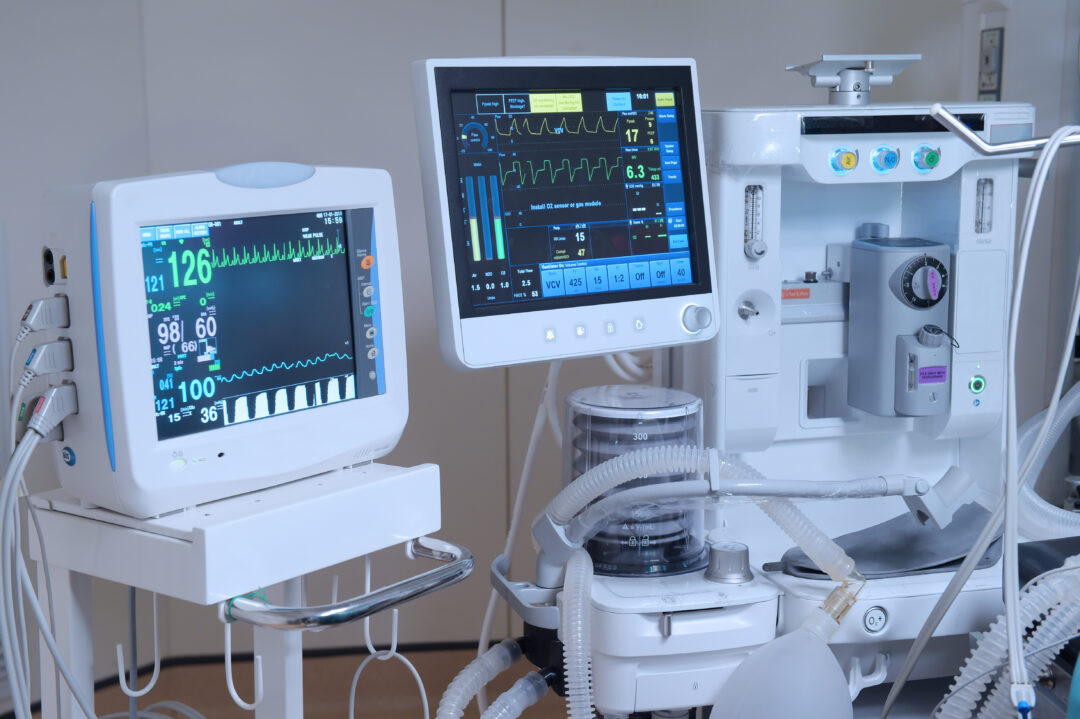
The medical industry is constantly evolving therefore change is inevitable, and how medical device industries manage change will definitely impact their business including, internal processes and the products that they design, develop, manufacture, and distribute into the market. Change management process in these companies will not only impact their work but also patients’ lives.
When the needs for change occur, you have to make sure all changes made are managed properly and everything is accounted for in your quality management system and the resulting documentation and records.
By definition, change management is the way the company manages modifications to products and processes within its business. There are many triggers for change throughout the lifecycle of a product or organization, including:
1- New or modified products, as well as any subsequent change to those products.
2- New or modified processes for how you conduct business as you right-size and grow your QMS.
3- New or modified controlled documents, such as templates, work orders, forms, etc., as well as any subsequent revisions made to those documents.
Also, there are some common change management challenges:
– Lack of visibility: You need an easy way to identify all the documents impacted by a proposed change order, a quick and error-free way so you don’t miss a thing. This requires either encyclopedic knowledge of the quality management system or a labor-intensive search through a large number of documents to find connections.
– Stuck waiting – working reactively: It might feel like you are starting over every time, spending time documenting, inking, and reviewing documents.
You will be losing money that you didn’t budget for from the hidden costs of redoing things. Recalls can cost millions and the safety of the product could be at risk.
– Increased risk: balancing speed, high quality, and lower costs, can make you feel under pressure. You cannot risk the safety of your products because you missed something in the change process. This often results in missed documents, requiring multiple change orders to be processed, or even worse, inconsistent and incomplete changes to be made to processes and product lines.
Although change triggers can vary, the change control process will involve the same steps:
1- Describe the change you’re making.
2- Specify the reason.
3- Identity what business outcomes will be affected.
4- Identify the people that need to be involved to assess and implement the change.
Every step in managing change effectively starts and ends with communication. No matter what type of change you are making could be in material used in the product or the logo on your packaging…, to make sure the change is managed efficiently and accounts for both stakeholder and compliance impact, you must facilitate clear communication between internal and external stakeholders being impacted by the change.
To describe the change you need to update your packaging specifications and requirements. When you define the reason for the change, you’ll cite the benefits — the proposed packaging is more durable and resilient to the temperature during shipping.
A brainstorming session could be very helpful in identifying the business outcome of the change that has to be made. It involves people from many departments putting their heads together to hypothesize the possible outcomes.
Therefore you need to identify the people who need to be involved, you must update any documents and work instructions associated with packaging to be reviewed by the relevant people in your organization.
Below you will find 5 key steps of the change management process:
1- Proposal and Evaluation
Whenever a need is identified or a change is proposed, the details such as change proposal, goals or objectives behind the proposed changes, justification, change classification into significant or non-significant, and action plans are documented and presented to the QA team and impacted department/s.
Proposed changes should be categorized into significant, non-significant or major and minor. Major changes impact the device safety or affect the product in the market, it requires extensive planning and documentation changes which may have a huge impact on the related process. Also, major changes require reporting to and getting approval from Health Authorities/ regulators before implementing the changes. Whereas minor changes could be more administrative or with limited impact on related processes.
2- Impact Assessment and Risk Assessment
Classification of change triggers the need for risk assessment. It is carried out to determine overall risk and the impact it can have on the impacted process. A risk mitigation strategy is drawn up by the QA and technical team.
3- Approval
After evaluation and risk assessment, the QA and designee will evaluate the need for proposed changes. After scrutiny, the proposed change will either be approved or rejected. The approved changes will proceed to implementation and the rejected changes will be recorded with a justification of rejection and then the record will be closed.
4- Implementation
After the approval, the implementation phase starts. The processes during this phase are carefully monitored and documented by the QA team and designated personnel. And documentation is crucial to ensure traceability in addition to training the impacted department members.
5- Post-Implementation Review
After the implementation, the QA team and the designee have to evaluate if the change implemented has met the desired goals and objectives. If the change has been successfully implemented the order is closed.
Major or minor changes can have implications for all the stakeholders of a business therefore it is important to ensure that the changes are communicated effectively.
What’s Next?
Now that you have learned about the change management for medical devices, learn about “The Essential Requirements of Medical Devices QMS“.
![]()
Leave a Comment
You must be logged in to post a comment.